|
By Alexander Bernstein
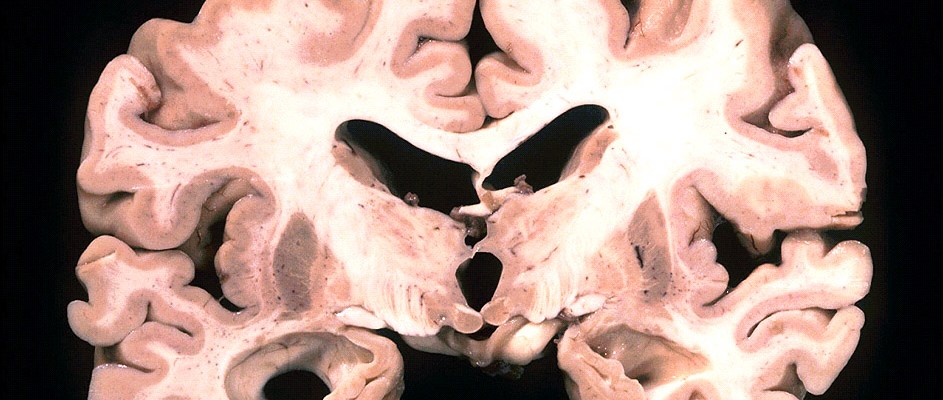
Defined by the Alzheimer’s Association as “decline in mental ability severe enough to interfere with daily life,” dementia is a crippling condition that affects more than 35 million people worldwide. One of the most common causes of dementia, Alzheimer’s disease has been, up until very recently, quite difficult to diagnose, typically requiring a brain autopsy to confirm the diagnosis. With the disease expected to affect some 115 million people by the 2050 and Alzheimer’s disease’s status as the only leading cause of death with no known effective method of treatment, developing new methods of early diagnosis is of the utmost interest to the scientific community. With that being said, research led by Claudio Soto of the Mitchell Center for Alzheimer’s Disease and Related Brain Disorders may be close to developing new diagnoses. By searching through the cerebrospinal fluid samples of 50 Alzheimer’s diseased patients, multiple people of normal cognition, and individuals with other neurodegenerative disorders, Soto and his colleagues from the University of Texas Medical School effectively demonstrated that prion-like diseases, which cause amyloid beta proteins to misfold, can be used to identify upcoming Alzheimer’s plaques. Utilizing a process resembling a polymerase chain reaction, the researchers have been able to identify minute quantities of these prion-like proteins. By amplifying hallmark proteins, Soto and his team were able to detect varying levels of such proteins in the urine and blood of animals, corresponding to symptomatic and presymptomatic stages of Alzheimer’s. Having established the oligomers’ link to the disorder, the next step is to clearly replicate these results in humans. With the current inability to effectively identify Alzheimer’s prior to the outbreak of symptoms, Soto’s research has heralded much attention as a potential break-through. Since these linked oligomers are believed to form years or even decades before the onset of symptoms, the detection of their presence could prove to be an effective method of predicting Alzheimer’s in the future. Although current work on humans still involves a painful spinal tap procedure, this method yielded remarkably accurate results for detecting misfolded proteins in patients already diagnosed with Alzheimer’s – effectively differentiating these individuals from those with other forms of dementia. Although it is believed that the first proteins to misfold primarily target the brain and cause toxic damage leading to Alzheimer’s, Soto and his fellow researchers believe that some of these proteins must travel to other parts of the body where they could be more easily detected. In a recent study, the scientists were able to effectively make Alzheimer’s predictions using this technology, with a 90% success rate for people with the disease, and a 92% success rate for those without the disease., Despite this initial success, the procedure is still in the early works. “This is just a proof of concept that this technology works,” Soto explains. Detection of Alzheimer’s in seemingly healthy patients has yet to be attempted, and proving the existence of the revealing oligomers in healthy patients provides problems as such individuals would probably decline painful tests. Further, threshold levels of the oligomers have yet to be determined since it is possible that healthy individuals may have miniscule amounts of the proteins as well. This being said, this research is groundbreaking, as it may provide the first plausible presymptomatic detection.
0 Comments
Leave a Reply. |
Categories
All
Archives
April 2024
|