|
By Alexander Bernstein
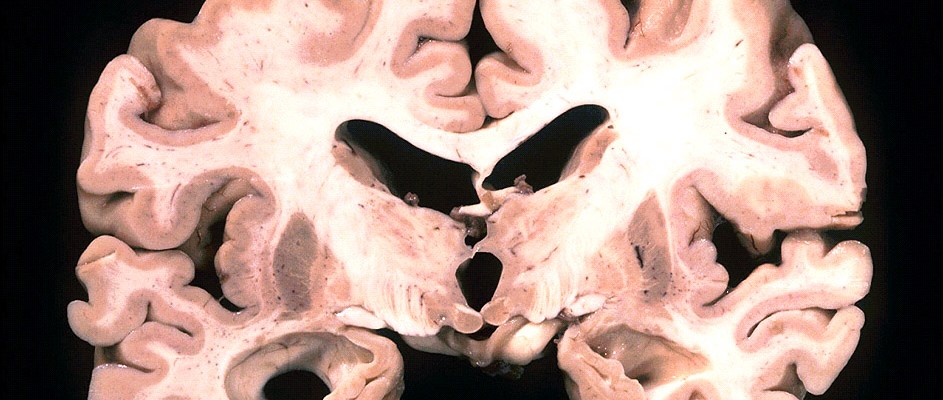
Defined by the Alzheimer’s Association as “decline in mental ability severe enough to interfere with daily life,” dementia is a crippling condition that affects more than 35 million people worldwide. One of the most common causes of dementia, Alzheimer’s disease has been, up until very recently, quite difficult to diagnose, typically requiring a brain autopsy to confirm the diagnosis. With the disease expected to affect some 115 million people by the 2050 and Alzheimer’s disease’s status as the only leading cause of death with no known effective method of treatment, developing new methods of early diagnosis is of the utmost interest to the scientific community. With that being said, research led by Claudio Soto of the Mitchell Center for Alzheimer’s Disease and Related Brain Disorders may be close to developing new diagnoses. By searching through the cerebrospinal fluid samples of 50 Alzheimer’s diseased patients, multiple people of normal cognition, and individuals with other neurodegenerative disorders, Soto and his colleagues from the University of Texas Medical School effectively demonstrated that prion-like diseases, which cause amyloid beta proteins to misfold, can be used to identify upcoming Alzheimer’s plaques. Utilizing a process resembling a polymerase chain reaction, the researchers have been able to identify minute quantities of these prion-like proteins. By amplifying hallmark proteins, Soto and his team were able to detect varying levels of such proteins in the urine and blood of animals, corresponding to symptomatic and presymptomatic stages of Alzheimer’s. Having established the oligomers’ link to the disorder, the next step is to clearly replicate these results in humans. With the current inability to effectively identify Alzheimer’s prior to the outbreak of symptoms, Soto’s research has heralded much attention as a potential break-through. Since these linked oligomers are believed to form years or even decades before the onset of symptoms, the detection of their presence could prove to be an effective method of predicting Alzheimer’s in the future. Although current work on humans still involves a painful spinal tap procedure, this method yielded remarkably accurate results for detecting misfolded proteins in patients already diagnosed with Alzheimer’s – effectively differentiating these individuals from those with other forms of dementia. Although it is believed that the first proteins to misfold primarily target the brain and cause toxic damage leading to Alzheimer’s, Soto and his fellow researchers believe that some of these proteins must travel to other parts of the body where they could be more easily detected. In a recent study, the scientists were able to effectively make Alzheimer’s predictions using this technology, with a 90% success rate for people with the disease, and a 92% success rate for those without the disease., Despite this initial success, the procedure is still in the early works. “This is just a proof of concept that this technology works,” Soto explains. Detection of Alzheimer’s in seemingly healthy patients has yet to be attempted, and proving the existence of the revealing oligomers in healthy patients provides problems as such individuals would probably decline painful tests. Further, threshold levels of the oligomers have yet to be determined since it is possible that healthy individuals may have miniscule amounts of the proteins as well. This being said, this research is groundbreaking, as it may provide the first plausible presymptomatic detection.
0 Comments
By Aditya Nair
One of the most baffling problems in science is that of consciousness. How exactly does our brain create consciousness? What brain mechanisms allow for the development of thoughts? Is consciousness a physical phenomenon? Probing the boundaries of our very existence itself, scientists have been pushing at this question for hundreds of years, edging ever closer to the truth. Many theories exist about the nature of consciousness, but one theory stands out as being particularly interesting and bizarre. A truly mind bending meld of physics and neuroscience, The Orchestrated Objective Reduction (Orch OR) model of consciousness – promoted by esteemed mathematician Sir Roger Penrose and anesthesiologist Stuart Hameroff of the Univerity of Arizona – suggests that consciousness is the result of collapsing wave functions at a quantum level. The world of quantum physics is known to be riddled with complicated and strange phenomena, such as particles that exist in two places at once and matter that exists and doesn’t exist at the same time. The quantum world is one of probability. This is obviously a far cry from the world of certainty that we have evolved to become extremely familiar with. The quantum world – the world of the extremely small – operates with a different set of rules than those of our everyday world. Dr. Hameroff, an anesthesiologist by trade, cites this as one of the primary reasons that he endorses the Orch OR model of consciousness. Hameroff postulates that a collapse of a quantum probability wave is the only possible non-physical phenomena that could account for the non-calculable process of consciousness. In other words, his theory finally provides a link between the world of physics and the world of thought, and it could begin to answer the fundamental questions of how physical processes in the brain can produce non-physical abstractions such as “thoughts,” and what “consciousness” actually is. Penrose, in his 1989 book The Emperor’s New Mind, lays out a similar argument: consciousness isn’t computable or algorithmic with classical physics; approaching the problem with quantum mechanics is the only way to arrive at an answer. Most experts in the field, however, disagree with Hameroff and Penrose. With advanced scientific and philosophical arguments that extend far beyond the scope of this article, both philosophers and neuroscientists refute the idea that quantum processes must be the root of consciousness. On the other hand, they argue, classical physics and electromagnetism are enough to bring about the types of interactions necessary to produce consciousness. Surely, the science of consciousness faces many years of refinement and evolution in the near future. What’s inspirational and exciting is that, while other fields of scientific research are facing deep budget cuts, interest in neuroscience seems to be growing and burgeoning. It is becoming a popular discipline, attracting the best and the brightest physicians, biologists, psychologists, philosophers, computer scientists, and yes, even physicists. By Alexander Bernstein
Especially following the recent mid 2000’s outbreak of Mad Cow Disease in Britain that saw nearly 200 thousand cattle and more than a hundred people infected, prions, the proteins associated with this disease, have a primarily served a boogeyman role in the media. Yet, as per a recent article published in Public Library of Science (PLOS) Biology, it appears that these proteins may have been misunderstood. Although some prions are in fact responsible for stimulating protein misfolding and thereby leading to destruction of appropriate cellular function in what is typically an irreversible and fatal process, the word done by Stowers Institute for Medical Research demonstrates that it is wrong to brand all prions under this same caustic banner. In his “The Proteins behind the Persistence of Memory”, Richard Robinson notes “At its most fundamental level, a memory is an increase in synaptic strength that persists over time.” With that in mind, he continues to point out in order to establish this synaptic effect specific protein function is required. From what is currently understood, those proteins are called Cytoplasmic Polyadenylation Element Binding (CPEB) proteins. Remarkably, these apparently fundamental proteins are, in their structure, very prion-like in that, as Robinson continues “Their conversion from monomers to oligomers, via stacking of their prion domains, is essential to the long-term maintenance of synaptic memory.” Indeed, it has been demonstrated that these proteins have no part in disease causing processes, as some of their cousins seem to do, but rather play a role in essential cellular function. Chief investigator of the PLOS Biology published research, Kausik Si points out that while much of the machinery in cells is altered and destroyed over time, these prion like proteins serve the role of stabilizing memories likely since prion folding is a self contained process and does not require external stimuli. Conducting their research on fruit flies, Si and his team demonstrated that the prion-like Orb2 protein is required for long-term memory since flies with a mutated version of this protein were able to learn new behaviors, but, unlike their counterparts with a normal version of the protein, were unable to practice these learned behaviors after a short period of time. As Si notes in the PLOS Biology article, “Beyond a day, the memories become unstable. By three days, the memory has completely disappeared.” Demonstrating the function of these proteins is just the beginning. Si and his colleagues are already planning to take their work further by examining how this prion function is regulated. Such a concept is intriguing because it is still unknown how prions are regulated. However, our fundamental understanding of memories and the fact that not all are long term seem to suggest that some regulatory processes must exist, and they determine when protein folding occurs. Thus far, Si has determined that Orb2 protein exists in two forms in the fruit fly – Orb2A and Orb2B. Orb2A is found in only certain specific neurons and degrades very quickly. Si explains that “When Orb2A binds to the more abundant form, it triggers conversion to the prion state, acting as a seed for the conversion.” This seed then is self-sustaining, and the team has demonstrated is partly regulated by a protein known as TOB, the binding of which stabilizes Orb2A and increases the prion nature. Since similar proteins have been found in both mice and humans, the study of their regulation and function is of utmost importance. By Ian MacArthur
Communication between members of different species belonging to the same biological class is complicated, as anyone who has ever attempted to talk to a household cat or dog could relate. It would seem, then, that communication between species belonging to different biological kingdoms, the second broadest classifiable category of species, would be all but impossible. Contrary to this intuition, researchers at the University of East Anglia (UEA) in the United Kingdom have found evidence of communication between our cells and the bacteria residing in our gut. It has long been known that beneficial bacteria living in the human intestine have played a vital role in human health and nutrition. Until now, though, evidence of communication between the organisms last sharing a common ancestor 3.5 billion years ago has been lacking. This communication appears to be related to the bacterial digestion of the phosphorus-containing compound phytate into phosphate and inositol, which are essential for human nutrition. The primary mode of phosphorus storage in plants is in the form of phytate. Humans lack the enzymes to digest phytate and therefore require intestinal bacteria to provide for its degradation before being able to use the essential nutrients the compound offers. The UEA researchers were able to identify a species of prominent gut bacteria as a producer of phytase, a family of phytate-degrading enzymes, and to elucidate the mechanism by which phytate is digested. The bacteria package phytase in special vesicles termed outer membrane vesicles (OMV) and secrete them into the lumen of the colon. There, the OMVs are able to uptake phytate and catabolize it into phosphate and inositol. The contents of the OMVs, including phytase, can then be taken up by colon epithelial cells. The UEA study suggests that phytase is not only significant for its catalytic activity in breaking down phytate but also for its role in communication between bacteria and colon epithelial cells. The investigators observed that calcium signaling in epithelial cells was altered after take-up of OMVs containing phytate. Calcium ions play diverse and important roles in cell signaling pathways. This implies that phytate, by influencing calcium signaling in epithelial cells, is able to influence the behavior of these cells. Although more research must be done to discover the precise details of how calcium signaling is affected by the enzyme, the UEA research has provided a starting point for further understanding the communication between bacteria and human cells. These findings are also significant from a medical standpoint. Human diseases caused by abnormal composition of intestinal bacteria may have a significant basis in how these bacteria communicate with epithelial cells. If future research is able to yield further details of how calcium signaling is affected by phytase uptake from OMVs, therapies may be developed to counteract abnormal bacteria-gut interactions. |
Categories
All
Archives
April 2024
|