|
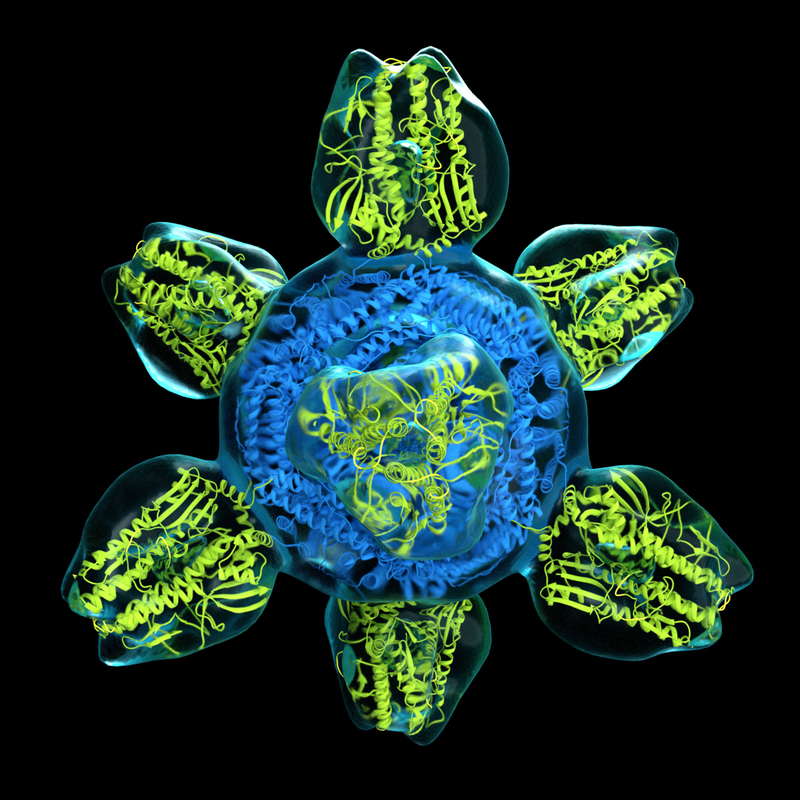
By Ethan Feng Proteins are important: imagine any process in your body, from digestion to immune response, and proteins are likely involved. So, it’s no surprise that scientists have long been working to understand the properties of these biochemical machines. In particular, grasping their structural details is crucial—think of proteins as puzzle pieces: a protein’s shape determines whether it can fit together and thus interact with other molecules. Ultimately, knowledge of these properties can provide insight into a protein’s function in living cells, which can help us solve important real-world problems in science and medicine. To illustrate the importance of structure, consider phenylketonuria (PKU), a genetic disease in which the protein responsible for breaking down the amino acid phenylalanine is either defective or missing. Patients must adhere to strict low-protein diets and, in bad cases, can suffer from intellectual disability and slowed growth. PKU has long been incurable; however, researchers investigating the protein defective in PKU patients, phenylalanine hydroxylase, recently mapped its structure for the first time. This crucial structural information gives scientists a clearer picture of what is wrong in PKU patients, opening the door to developing more effective treatments. As we can see, protein structures are important. Unfortunately, while researchers conquered this particular case, studying protein structure is generally very difficult—especially when it comes to imaging. A single protein molecule is typically less than 10 nanometers across—equivalent to the width of a hair divided by 100, and then by 100 again! Moreover, at normal temperatures, molecules vibrate and twist extremely quickly, making it difficult to pinpoint any precise structural details beyond a mere blurry blob. Traditionally, to obtain structural information about proteins, scientists relied on a method known as X-ray crystallography. This technique involves arranging a protein into a crystal structure, which locks the molecules into a steady position and prevents them from wiggling around too much, making it easier to see them clearly. Then, scientists bombard the crystal with X-ray radiation, and the pattern in which the rays diffract helps determine the arrangement of the atoms. While undeniably a monumental tool in biochemistry, X-ray crystallography is limited in scope, as many proteins are too flexible and disordered to form stable crystal structures, making them impossible to analyze with this technique. To solve this issue, biochemists have revolutionized the field with a new, versatile technique known as cryogenic electron microscopy--or cryo-EM for short—which can be applied even to more unwieldy molecules. To tackle the issue of molecules wiggling around too much, instead of relying on crystallization, cryo-EM takes a different approach: it harnesses temperature. In this method, scientists cool a protein sample rapidly by plunging it into liquid ethane at -196°C. Temperature can be thought of as a measure of how quickly the molecules within a substance are moving, so by lowering the sample’s temperature substantially, the protein’s movements slow down to the point that they are effectively frozen in time, visibly unmoving. Thus, freezing the protein means the usual twisting and turning is no longer a problem! Then, the cold sample is placed into an electron microscope for imaging: a beam of electrons is shot at the sample and sensors detect how the samples scatter the electrons, allowing a computer program to piece together a high-resolution 3D model, down to near-atomic resolution. A key advantage of cryo-EM is that, unlike X-ray crystallography, it does not require crystal structures. A crystal, by its very nature, consists of molecules that are highly uniform. Therefore, when doing X-ray crystallography, it is difficult to observe more than just one state or conformation of the molecule of interest. This is a limitation because structural biochemists are oftentimes interested in the many ways a protein can fold, as well as how it moves between these states. In the words of the Royal Swedish Academy of Sciences, images from X-ray crystallography “are like black and white portraits from early cameras—their rigid pose reveals very little about the protein’s dynamics.” Cryo-EM overcomes this limitation: as the sample rapidly freezes, each molecule is largely independent and is not locked into any particular state, meaning that any given cryo-EM sample typically contains a wide variety of states and conformations. To further illustrate why this is useful, imagine how if you were given various frames of a person running, you could roughly reconstruct the runner’s motions—in the same way, using the various states of a protein observed via cryo-EM, scientists can infer the ways the protein moves. What this essentially means is that cryo-EM not only takes still images but can also produce movies of a molecule’s movements! This has helped scientists understand how proteins fold and unfold and even how reactions occur in real-time. Researchers are currently developing new and more targeted methods to capture rare and transient states of proteins that have never been visualized before. Today, cryo-EM continues to be monumental in improving scientists’ understanding of proteins. Its popularity has been rising rapidly—a database of protein structures determined by cryo-EM acquired its 10,000th entry earlier this year, including nearly 3,000 this year alone. In fact, the influence of cryo-EM was deemed so widespread and impactful that in 2017, the Nobel Prize in Chemistry was awarded to the three biochemists who pioneered the technique, including Columbia’s very own Joachim Frank. Furthermore, the impact of cryo-EM reaches far beyond the confines of academia and theory: recently, researchers at the MRC Laboratory of Molecular Biology used cryo-EM to determine the structure of gamma secretase, a protein crucial to understanding how to treat Alzheimer’s disease whose structure had long been indiscernible using other methods. By helping researchers push forward their grasp of protein structure and dynamics, this ~cool~ technique will help us strive towards better treatments for disease.
0 Comments
Leave a Reply. |
Categories
All
Archives
April 2024
|