|
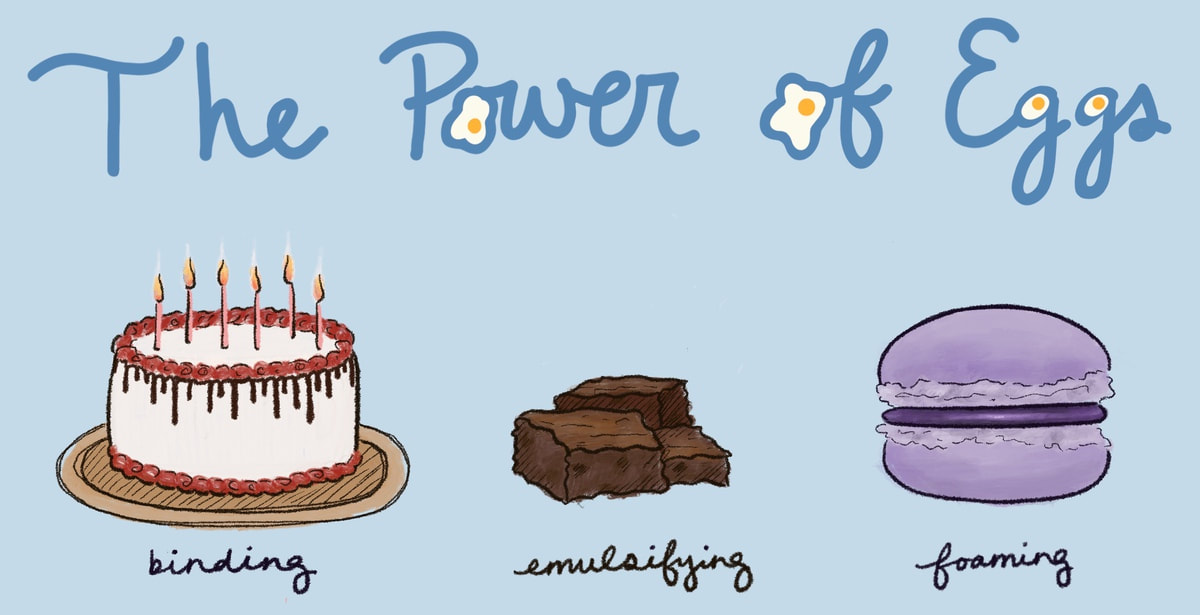
By Shivani Tripathi A fudgey brownie that melts in your mouth. Crispy, sweet macarons for a snack. Your red-velvet birthday cake just waiting to have its candles blown. Whether it be to celebrate another year of life or to satisfy 3 AM cravings, dessert is one of the most joyful aspects of the human experience. Though dessert remains a global constant, there is a revolution brewing in the recipes of our favorite sweets. People are increasingly opting to replace an essential ingredient in desserts: eggs. Reasons for doing so include (but are not limited to) environmental sustainability, allergies, and cholesterol problems. Many worry that excluding eggs will result in their desserts being flat and lackluster. Although egg substitutes do not entirely emulate the polyfunctionality of an egg, there are a variety of egg replacements that can contribute to making delicious sweets. The three most important functions of eggs in desserts are emulsion, foaming ability, and binding. Emulsion is defined as the combination of two substances that are not naturally miscible. Eggs contain emulsifiers, molecules with one hydrophobic (water-resistant) end and one hydrophilic (water-loving) end. Their hydrophobic and hydrophilic qualities enable the emulsifiers to bond with both fat and liquid, preventing both substances from separating. To make desserts such as brownies, we use two immiscible ingredients: fat (such as butter) and liquid (such as milk.) Without an emulsifier, these two vital ingredients wouldn’t mix properly, resulting in a grainy, flat, and uneven baked good. Luckily, there are a variety of plant-based emulsifiers which can salvage our brownies. Plant-derived lecithins, aquafaba, and algal flour all contain emulsifiers. Lecithin is a multipurpose fat, its functions ranging from emulsification to treating depression. Aquafaba is the liquid leftover from soaked chickpeas, containing emulsifiers and proteins similar to eggs. Algal powder is composed of monoglycerides, diglycerides, and phospholipids which serve as emulsifiers. Regardless of which replacement you choose, each option is adept in emulsifying your ingredients. Emulsion is not the only sweet thing about eggs. Another integral function of eggs is their ability to stabilize foam. Desserts like macarons owe their light, and airy structure to egg whites. Whipping egg whites denatures the egg proteins, trapping air bubbles. In baking, these egg proteins bond with wheat proteins to create a rigid network of air bubbles that expand upon being heated. Even after being cooled, the strong protein network enables the desserts to retain their airy structure. This enables macarons to have a crispy mouthfeel. A replacement for egg whites can be found in a substance that usually goes to waste: aquafaba. High in protein, starch, and fiber, aquafaba is similar to egg whites chemically. To use aquafaba as a foam stabilizer, you just need to add some cream of tartar. The cream of tartar elevates the stability of the aquafaba. Then, whip the solution at a high speed until stiff peaks form. And voila! You can use the foam to make a variety of desserts that were once thought impossible to veganize. Binding is the third, and arguably most important, use of eggs in baking. Without binding, desserts such as your red velvet birthday cake would cease to stand. An egg’s binding ability can be attributed to its gelatinous and coagulating properties. When triggered by heat, egg proteins denature and form a three-dimensional gel consisting of water and protein, resulting in a cuttable texture. An egg’s gelatinous properties can be replicated by ground flax and chia seeds. You can mix a teaspoon of either seed into three teaspoons of water. After approximately 20 minutes, the mixture will have become thick and gelatinous. Due to their high protein and fiber concentrations, flax and chia eggs are highly effective at binding baked goods. Another way that eggs bind baked goods is through coagulation. Coagulation occurs when the over 40 different proteins in an egg denature and bond with each other, transforming the liquid egg into a solid. This process provides sweets such as cakes with a firm structure. Products such as VeganEgg and JustScramble can coagulate like eggs, but only the former can be used in desserts. Despite the variety of plant-based egg substitutes, there is not yet an alternative that possesses all of the qualities of an egg. Even then, anecdotal evidence suggests that the difference in taste between eggless and egg-containing desserts are subtle, if any. Moreover, even if eggless desserts taste slightly different, different doesn’t mean worse. Humanity possesses a vast array of thoughts, motivations, and desires. Our dessert preferences merely reflect our diversity.
0 Comments
Leave a Reply. |
Categories
All
Archives
April 2024
|