|
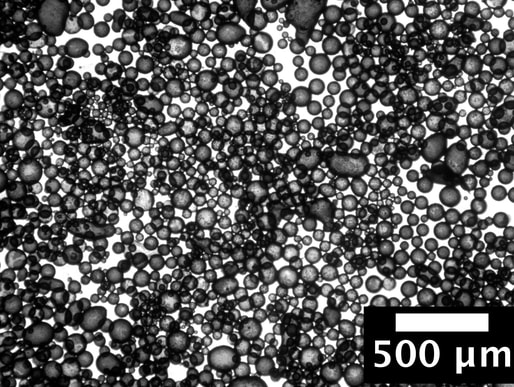
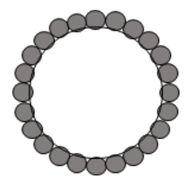
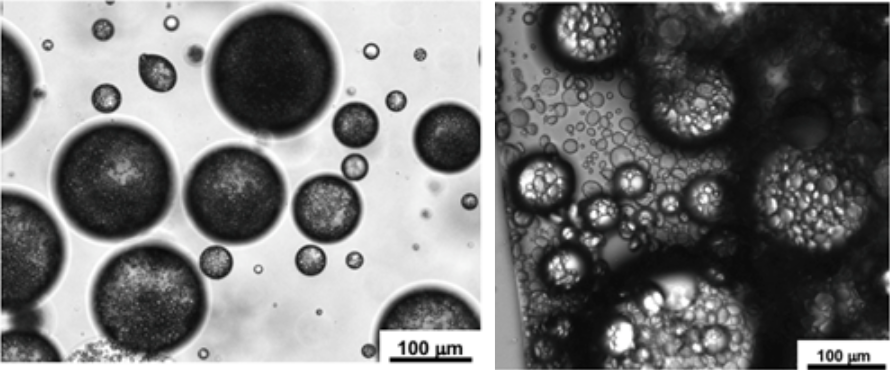
By Boyuan Chen “Try it yourself, just once,” said a voice, low and hoarse. It was Grandma, smiling. My Grandma, a traditional Chinese countrywoman, always preferred to use the wastewater left from boiling noodles to wash dishes, a practice I have long held doubts about. 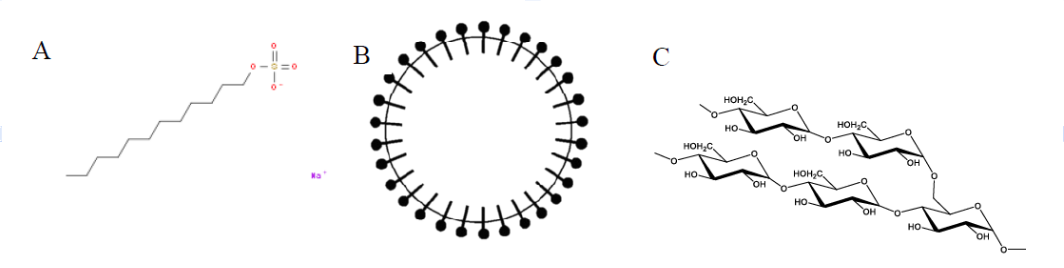 As a chemistry enthusiast, I knew by heart that floury water contains mostly starch, a polysaccharide macromolecule that does not qualify as a surfactant, the class of chemicals that common dish soap contains. Generally, dish soap works by the amphiphilicity of the surfactant molecules; this chemical property—having a head attracted to water and a tail attracted to oil—allows these molecules to bind residual oil to running water. As a result, a stable mixture of water and oil, or an emulsion, is formed, further enabling water to wash away the residual oil. How on earth, then, can floury water serve as the role of dish soap? Figure 1. The molecular structure of a common molecular surfactant, sodium laurylsulfonate (A), and its arrangement around oil droplets in water (B). (C) is what a starch molecule is made of. Grandma beamed at me understandingly, anticipating my qualms. "It's ancient wisdom,” she chuckled. “It works." This was Grandma’s habitual answer to my scruples, but sincere as it was, my doubts remained So I started washing. Dipping dishes into the floury water, I wanted to prove my knowledge of chemistry with my own hands. Yet, I was astonished—the thick oil miraculously vanished, leaving the porcelain clean as new. I was as ashamed as I was shocked. Later, I also learned that some families also use the wastewater left from rinsing rice to wash dishes, so starch surely works! But the question remains: how? After some research, I found out that in addition to molecular surfactants, there exists yet another type of material that may bind water and oil together: Pickering emulsifiers. In these cases, particles with sizes ranging from hundreds of nanometers to a few micrometers function as stabilizers in the water-oil mixture. These particles, which also exhibit both water and oil affinity, may spontaneously attach to the water-oil interface due to surface energy (the same reason that water striders can walk on the surface of water). Thus, a barrier around each oil droplet is formed, allowing them to mix with water as shown in Figure 2. Figure 2. The diagram of Pickering emulsion. Natural starch happens to be composed of such particles. Starch is naturally produced and stored in plant cells as small granules ranging from 1 to 100 micrometers in diameter. This fact precisely explains why rice-rinsing water works as a cleaning agent. Rice starch granules have a volume moment mean diameter (the technical term for diameter in fluid mechanics and colloidal science) of 4.61μm, which would easily fall into the size range of Pickering emulsifiers. Therefore, native rice starch can serve as a very efficient emulsifier. Things only get more interesting when it comes to noodle-cooking water, the one Grandma uses. Wheat starch granules have a volume moment mean diameter of 22.88μm, which would be way too large to qualify as a Pickering emulsifier. Upon heating, however, wheat starch granules start to swell and become sticky. In the floury water, the swollen wheat starch granules acquire a larger surface area, which increases the interfacial force on each granule and decreases density, lessening the destabilizing effect of gravity. The combined result of both factors is a stable emulsion. Figure 3. Emulsions stabilized by rice starch and wheat starch.
This seemingly magical practice not only challenges our preexisting notions in science, but also holds significant implications for an eco-friendly alternative to traditional surfactants. Rice-rinsing water and floury noodle water are readily available in kitchens and in factories yet are usually disposed of carelessly by those unaware of its potential. They are completely sustainable and biodegradable, which means that starch-based detergents would not only be cheaper but also greener than the non-biodegradable detergents based on molecular surfactants, the production of which also relies on the petrol industry. Beyond cleaning dishes, they may also replace traditional surfactants in personal care products like body wash, shampoo, and certain emulsion-based cosmetics. Grandma’s cleaning formula works, after all.
0 Comments
Leave a Reply. |
Categories
All
Archives
April 2024
|