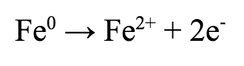By Elifsu Gencer Although fresh water is essential for life, it remains a limited resource threatened by physical, chemical, biological, and radiological contaminants. According to the World Health Organization (WHO), 30% of the global population lack access to drinkable water at home, and 60% lack safely managed sanitation. Moreover, the WHO estimates that by 2025, half of the world’s population will be living in water-stressed areas. Industrial processes have especially exacerbated water scarcity issues by generating wastewater (water that has been contaminated by human use) and discharging large concentrations of toxic pollutants into groundwater, the water found in cracks and spaces beneath Earth’s surface and which serves as a valuable drinking source for millions around the world. Accordingly, wastewater and groundwater remediation processes are growing increasingly important, such as air sparging to volatilize contaminants and the pump-and-treat method that involves pumping subsurface contaminated water for above-ground treatment. These processes have been continuously innovated to enhance contaminant removal efficiencies, decrease costs, and increase environmental soundness. In particular, the method of using zero-valent iron (ZVI) has attracted considerable attention in recent years. Although zero valent aluminum or magnesium has been used since the late 1980s to dechlorinate chlorinated organic compounds, ZVI’s functionality had been largely overlooked due to its relatively slower reaction rate. However, this controlled reactivity became an advantage in ZVI’s application to groundwater systems in the early 1990s and since then, the use of ZVI in water remediation has gained great popularity. ZVI’s ability to remove contaminants is a product of its excellent reducing power, its ability to adsorb (adhere to) heavy metals and metalloids, and its magnetic properties, which allow for easy separation of ZVI from water by magnets. The oxidation potential of ZVI is +0.44 V, indicating that it behaves as a reducing agent by donating electrons in the presence of species with relatively more positive reduction potentials. The half-reaction describing the oxidation of ZVI is: Many heavy metals, including common water contaminants such as lead, mercury, and chromium, have larger reduction potentials, which allow them to be reduced and precipitated in the presence of ZVI. Additionally, ZVI can react with dissolved oxygen and water in a series of reactions to produce hydroxyl radicals via a Fenton reaction. The produced hydroxyl radicals have strong oxidizing capabilities and have been shown to degrade organic and inorganic contaminants.
In addition to its strong reducing power, ZVI has been successful in water remediation due to its high total surface area of a material per unit of mass, otherwise known as the specific surface area, and its resulting ability to adhere to certain contaminants to induce reduction and precipitation. As such, there is great interest surrounding the application of nanoscale zero-valent iron (nZVI) particles, which range from 10 to 100 nanometers in diameter due to their increased surface area. nZVI provides a greater, yet still controlled, reactivity compared to ZVI microparticles. Although nZVI particles have exhibited an effective and versatile ability to remove organic and inorganic contaminants, their application poses challenges, such as their propensity to aggregate amongst themselves due to their Van der Waals and magnetic attraction forces. Aggregation decreases the particles’ mobility and reduction capacity, ultimately lowering the contaminant removal efficiencies. Aggregation issues have been addressed by the immobilization of nZVI onto supports—solid porous materials such as carbon, resin, kaolinite, and membranes—without decreasing the reduction ability of nZVI. For example, in 2010, a team of researchers from Fujian Normal University in China investigated the application of kaolin-supported nZVI to the removal of Pb(II) from aqueous solutions under various conditions, including concentration of Pb(II), pH, and contact time. The study demonstrated that 90.1% of the metal ions from aqueous solution containing 500 mg/L of Pb(II) could be removed within an hour using 5 g/L of kaolin-nZVI. The material’s reusability was also assessed, demonstrating that 10 g/L of kaolin-nZVI could be reused up to five times for every initial concentration of 50 mg/L of Pb(II) in solution, with an impressive removal efficiency of 80.5% by the fifth reuse. Aggregation has also been addressed by diluting Van der Waals and magnetic attraction forces with the introduction of another inert, non-magnetic metal onto the surface of ZVI particles to create what are known as bimetallic particles. These bimetallic nZVI particles can be synthesized by immersing the freshly prepared nZVI particles in a solution of the second metal salt, such as those of palladium, nickel, or platinum. In addition to overcoming the aggregation of regular nZVI particles, these bimetallic nanoparticles offer the benefit of enhanced reduction rates due to catalytic mechanisms by the second metal. More specifically, the bimetallic particles form a galvanic couple due to their dissimilar reduction/oxidation potentials and subsequently allow for catalytic electron transfer and hydrogenation. Bimetallic nZVI particles also reduce the deposition of toxic intermediate products, such as vinyl chloride, onto particles’ surfaces. NZVI/copper bimetallic nanoparticles (NZVI/Cu0) have been shown to be particularly effective in the reduction of contaminants like phosphorus, p-nitrophenol, and nitrobenzene, most likely due to the high potential difference between Fe and Cu that promotes catalysis of Fe0 oxidation, even under neutral conditions. Another application of ZVI is the creation of ZVI-permeable reactive barriers (ZVI PRBs), which have become prominent within in situ remediation technology. ZVI PRBs can be installed along the path of contaminated groundwater and are capable of degrading and immobilizing contaminants passing through the barrier. The advantages of ZVI PRBs include low maintenance costs and their ability to remove many different contaminants, including chlorinated organic compounds, nitrate, heavy metals, and even explosives at RDX- and TNT-impacted sites. Some of their disadvantages, however, include construction challenges posed by groundwater depth or geologic media and their inability to remove contaminants beyond the barrier. Research is currently being conducted to address these disadvantages and to provide insight into the long term performance of ZVI PRBs. Furthermore, there is a need for large-scale in situ studies that compare lab- and commercial-scale applications at contaminated sites. Although the use of ZVI in water remediation presents itself as a promising emerging technology, much more research is needed in order to understand how it specifically functions with different contaminants. Removal mechanisms can be complex, depending on the contaminant, and may include reduction/oxidation, adsorption, surface precipitation, surface complexation, and co-precipitation processes that can occur either simultaneously or sequentially. A better understanding of these mechanisms would facilitate the removal of a much wider range of contaminants. Despite these uncertainties, ZVI has great potential in addressing water scarcity, as well as protecting people and the environment against the potential adverse effects of contaminants.
0 Comments
Leave a Reply. |
Categories
All
Archives
April 2024
|